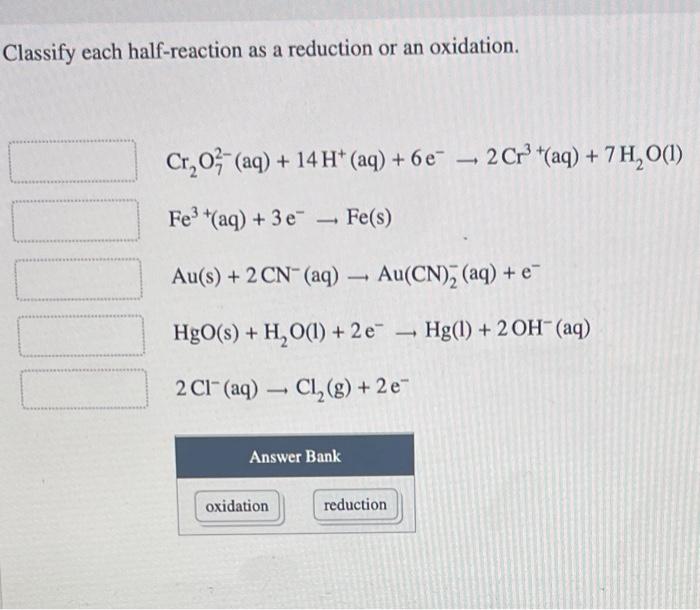
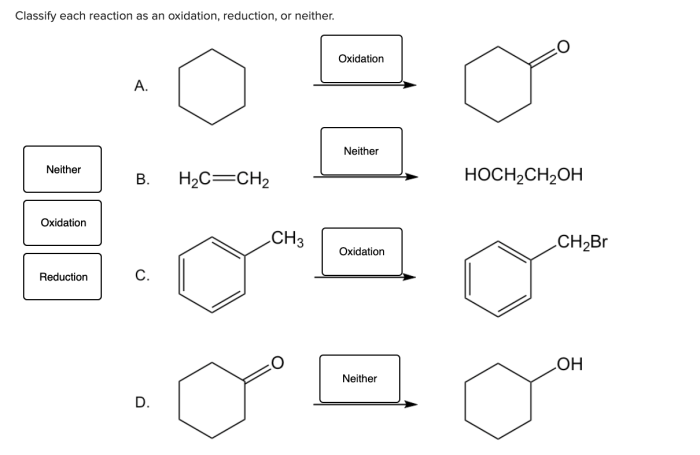
Classify each half-reaction as a reduction or an oxidation – In the realm of chemistry, oxidation-reduction reactions play a pivotal role. Understanding how to classify half-reactions as oxidation or reduction is crucial for comprehending these intricate processes. This article delves into the fundamental concepts of oxidation and reduction, providing a comprehensive guide to identifying and categorizing half-reactions.
By elucidating the transfer of electrons and changes in oxidation states, we establish a solid foundation for understanding the criteria used to distinguish between oxidation and reduction half-reactions. Through illustrative examples and clear explanations, this article empowers readers to confidently classify half-reactions, unlocking a deeper comprehension of redox reactions.
Understanding Oxidation and Reduction
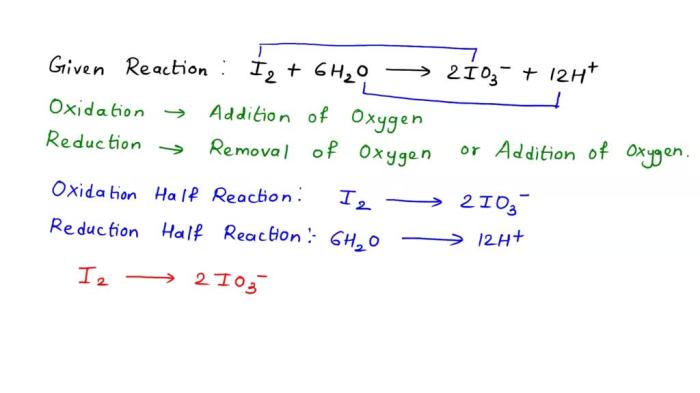
Oxidation and reduction are fundamental chemical processes involving the transfer of electrons between reactants. Oxidation refers to the loss of electrons, resulting in an increase in oxidation state, while reduction refers to the gain of electrons, leading to a decrease in oxidation state.
Classifying Half-Reactions, Classify each half-reaction as a reduction or an oxidation
In a chemical equation, a half-reaction represents a single oxidation or reduction process. To classify a half-reaction, examine the following criteria:
- Oxidation Half-Reaction:Loss of electrons, increase in oxidation state.
- Reduction Half-Reaction:Gain of electrons, decrease in oxidation state.
Oxidation Half-Reactions
Examples of oxidation half-reactions include:
- Fe 0→ Fe 2++ 2e –(Loss of 2 electrons, oxidation state increases from 0 to +2)
- Zn → Zn 2++ 2e –(Loss of 2 electrons, oxidation state increases from 0 to +2)
Reduction Half-Reactions
Examples of reduction half-reactions include:
- Cu 2++ 2e –→ Cu 0(Gain of 2 electrons, oxidation state decreases from +2 to 0)
- Fe 3++ e –→ Fe 2+(Gain of 1 electron, oxidation state decreases from +3 to +2)
Balancing Half-Reactions
Balancing half-reactions involves adjusting the number of electrons lost or gained to ensure the overall reaction is balanced. This is achieved by:
- Determining the change in oxidation state for each reactant.
- Using oxidation numbers to calculate the number of electrons transferred.
- Adjusting coefficients to balance the electrons lost and gained.
Question & Answer Hub: Classify Each Half-reaction As A Reduction Or An Oxidation
What is the difference between oxidation and reduction?
Oxidation involves the loss of electrons, while reduction involves the gain of electrons.
How can I identify oxidation half-reactions?
Oxidation half-reactions show an increase in oxidation state or the loss of electrons.
What is the significance of classifying half-reactions?
Classifying half-reactions allows us to balance redox reactions and understand the overall electron transfer process.