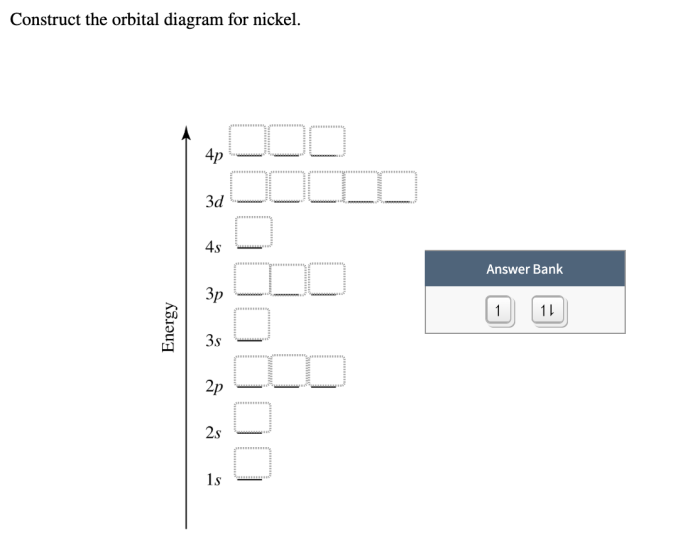
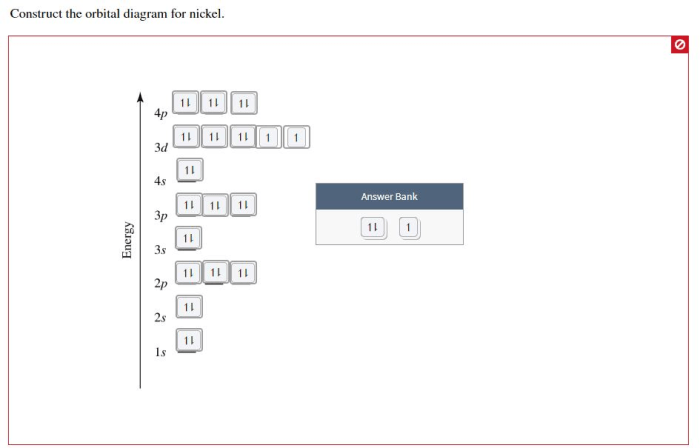
Construct the orbital diagram for nickel. – Construct the orbital diagram for nickel, a journey into the captivating realm of quantum mechanics. This guide unravels the intricacies of electron configurations, orbital diagrams, and their profound implications in chemistry.
Delve into the electronic structure of nickel, deciphering the distribution of electrons across energy levels. Discover the principles governing orbital filling and the significance of electron spin. Witness the construction of an orbital diagram, a visual representation of the electron arrangement, using the Aufbau principle and Hund’s rule.
Electron Configuration of Nickel
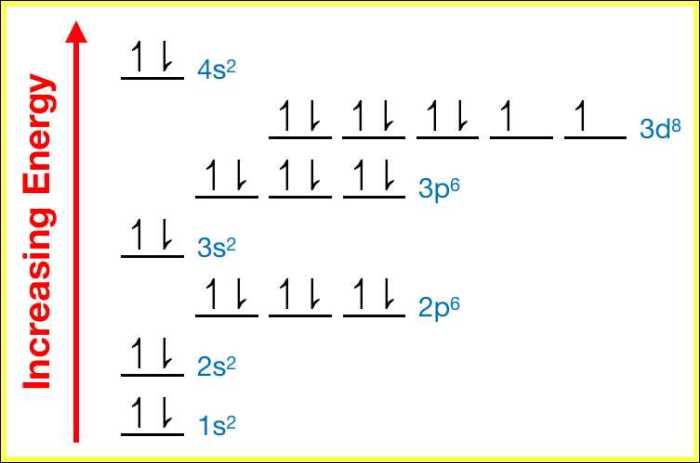
The electron configuration of an element describes the distribution of its electrons in atomic orbitals. An orbital diagram is a visual representation of this electron configuration, showing the energy levels and orbitals occupied by the electrons.
Electron Configuration of Nickel
The electron configuration of nickel (Ni) is 1s 22s 22p 63s 23p 63d 84s 2. This means that nickel has two electrons in the 1s orbital, two in the 2s orbital, six in the 2p orbital, two in the 3s orbital, six in the 3p orbital, eight in the 3d orbital, and two in the 4s orbital.
Constructing the Orbital Diagram
To construct an orbital diagram for nickel, we can use the following steps:
- Draw the energy levels as horizontal lines. The energy levels increase in energy from bottom to top.
- Label each energy level with its corresponding quantum number (n).
- For each energy level, draw the orbitals as circles. The number of orbitals in each energy level is equal to 2n2.
- Fill the orbitals with electrons, following the Aufbau principle and Hund’s rule.
Orbital Notation, Construct the orbital diagram for nickel.
Orbitals are represented in an orbital diagram using the following notation:
- The energy level is represented by the principal quantum number (n).
- The shape of the orbital is represented by the angular momentum quantum number (l).
- The orientation of the orbital in space is represented by the magnetic quantum number (m l).
- The spin of the electron is represented by the spin quantum number (m s).
Essential FAQs: Construct The Orbital Diagram For Nickel.
What is the electron configuration of nickel?
[Ar] 3d8 4s2
How many electrons are in the 3d orbitals of nickel?
8
What is the Aufbau principle?
Electrons fill orbitals in order of increasing energy levels.
What is Hund’s rule?
When filling orbitals of equal energy, electrons occupy separate orbitals with parallel spins before pairing.