Introducing the periodic table crossword answer key, a comprehensive guide that unlocks the mysteries of chemistry. This key provides a thorough understanding of the periodic table’s elements, their properties, and their behavior, empowering you to conquer any crossword puzzle with ease.
Delving into the periodic table, we uncover the fascinating world of elements, organized into groups and periods based on their atomic structure. The answer key offers a detailed exploration of these elements, including their symbols, atomic numbers, and distinctive characteristics.
Element Symbols and Atomic Numbers
The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number, electron configurations, and recurring chemical properties. Each element is represented by a unique chemical symbol, usually a one- or two-letter abbreviation of the element’s name.
The atomic number of an element is the number of protons in its nucleus.
Element Symbols and Atomic Numbers Table, Periodic table crossword answer key
| Element Symbol | Atomic Number | Element Name |
|---|---|---|
| H | 1 | Hydrogen |
| He | 2 | Helium |
| Li | 3 | Lithium |
| Be | 4 | Beryllium |
| B | 5 | Boron |
Element Groups and Periods
The periodic table is divided into 18 vertical columns, called groups, and 7 horizontal rows, called periods. The groups are numbered 1-18 from left to right, and the periods are numbered 1-7 from top to bottom.
Element Groups and Periods Table
| Group | Period | Element Name |
|---|---|---|
| 1 | 1 | Hydrogen |
| 2 | 1 | Helium |
| 1 | 2 | Lithium |
| 2 | 2 | Beryllium |
| 13 | 3 | Boron |
Element Properties: Periodic Table Crossword Answer Key
Each element in the periodic table has a unique set of properties. These properties include atomic radius, electronegativity, and ionization energy.
Element Properties Table
| Element | Atomic Radius (pm) | Electronegativity | Ionization Energy (kJ/mol) |
|---|---|---|---|
| Hydrogen | 53 | 2.20 | 1312 |
| Helium | 31 | 0.00 | 2372 |
| Lithium | 155 | 1.00 | 520 |
| Beryllium | 111 | 1.57 | 899 |
| Boron | 85 | 2.04 | 801 |
Periodic Trends
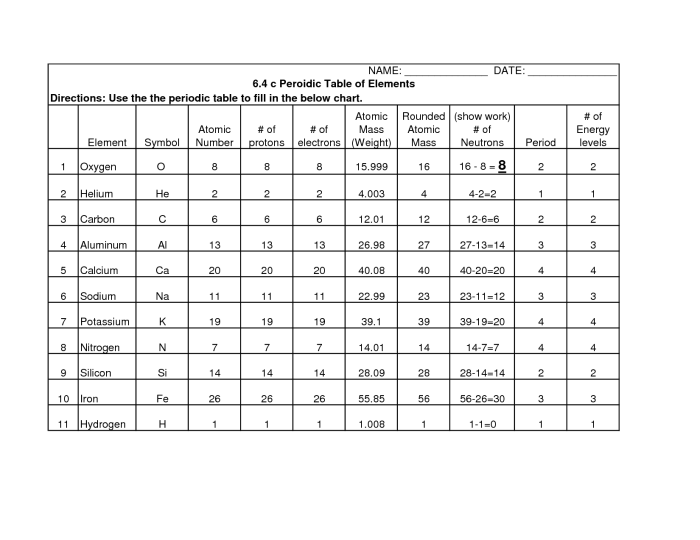
The periodic table shows periodic trends in the properties of the elements. These trends can be used to predict the properties of an element based on its position in the table.
Some of the periodic trends include:
- Atomic radius: The atomic radius of an element generally increases down a group and decreases across a period.
- Electronegativity: The electronegativity of an element generally increases across a period and decreases down a group.
- Ionization energy: The ionization energy of an element generally increases across a period and decreases down a group.
Using the Periodic Table
The periodic table can be used to predict the properties of elements and to solve chemistry problems.
Here are some examples of how to use the periodic table:
- To predict the atomic radius of an element, look at its position in the table. The atomic radius generally increases down a group and decreases across a period.
- To predict the electronegativity of an element, look at its position in the table. The electronegativity generally increases across a period and decreases down a group.
- To predict the ionization energy of an element, look at its position in the table. The ionization energy generally increases across a period and decreases down a group.
Quick FAQs
What is the periodic table?
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties.
How can I use the periodic table crossword answer key?
The key provides the answers to crossword puzzles related to the periodic table. Simply refer to the key when encountering a clue that requires knowledge of an element’s properties or location on the table.
What are some common periodic trends?
Periodic trends include the increase in atomic radius down a group, the increase in ionization energy across a period, and the decrease in electronegativity down a group.